


Areas of Activity
News
Image gallery
Documentation Center
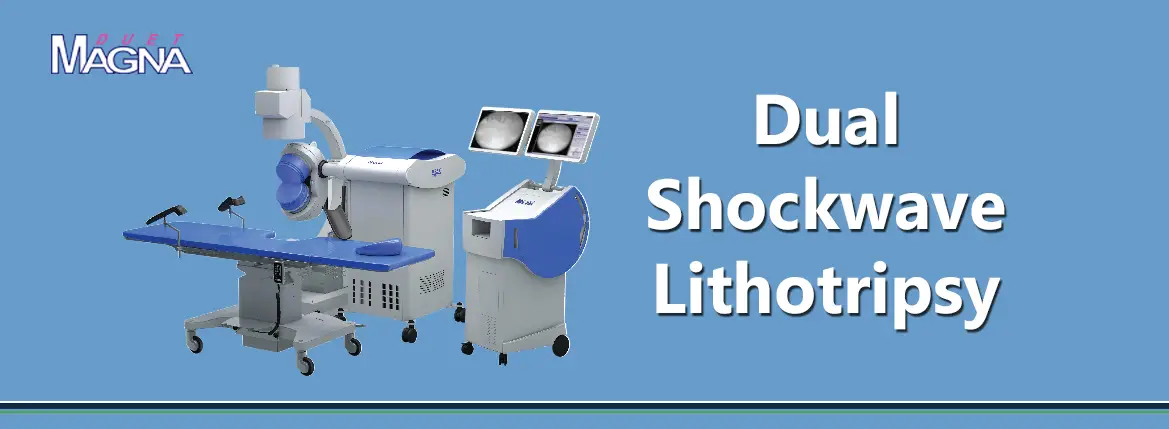
DirexGroup is dedicated to providing urologists worldwide with innovative high-technology devices.
DirexGroup network includes distributing companies and direct service centers in the US, Mexico, Brazil, Argentina, Italy, Germany, United Kingdom, France, Spain, Russia, China, Korea, Japan and India. The group is one of the global leaders in Shockwave Lithotripsy utilizing pioneering breakthrough technologies in this field since the mid 1980s. To-date, the group supports urologists in over 70 countries, relying on its network of companies and alliances worldwide.






